I. Project Background: Why an Integrated Engineering Approach Is Essential
Hospital pathology departments are widely recognized as the “gold standard” of clinical diagnosis. The quality and reliability of pathology services directly affect diagnostic accuracy, clinical decision-making, and patient outcomes.
However, a pathology department is not a single-function space—it is a highly complex engineering system involving:
- Biological safety and occupational protection
- Control of hazardous chemical vapors
- Clean environment and pressure gradient management
- Stable operation of precision instruments
- Coordination of personnel, specimen, and material workflows
In practice, projects based on fragmented design, isolated equipment procurement, or poorly coordinated ventilation systems often lead to:
- Unstable pressure differentials and airflow disorder
- Risk of biological and chemical leakage
- Mismatch between ventilation systems and pathology equipment
- High energy consumption and difficult maintenance
- Challenges in infection control, biosafety, and environmental compliance acceptance
For these reasons, pathology departments must be planned and delivered as integrated engineering systems, with the ventilation and exhaust system as the central safety backbone—fully coordinated with architecture, cleanroom design, process equipment, and operational requirements.
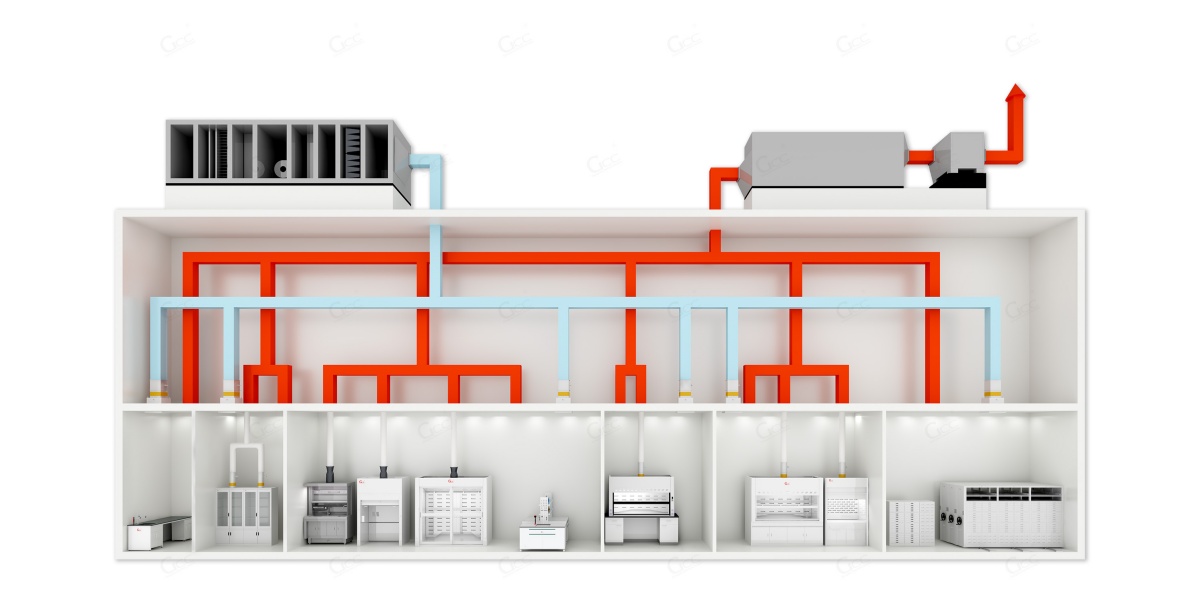
II. Core Philosophy of the Integrated Solution
Our pathology department integrated engineering solution is built around the ventilation and exhaust system as the primary safety framework, forming three interrelated systems:
1. Risk-Oriented System Design
Targeting biological aerosols, chemical vapors, and cross-contamination risks through a strategy of source containment, spatial isolation, and directional airflow control.
2. Functional Zoning and Engineering Coordination
Clear definition of contaminated zones, semi-contaminated (buffer) zones, and clean zones, with ventilation systems delivering verifiable pressure gradients, not just conceptual zoning.
3. Lifecycle-Oriented Delivery
Beyond meeting initial inspection and acceptance requirements, the solution prioritizes long-term operational stability, energy efficiency, maintainability, and future scalability.
III. Integrated Engineering Solutions by Functional Area
1. Specimen Receiving and Grossing Area
(High-Risk Contaminated Core Zone)
Engineering Objective:
Achieve effective source capture and zero leakage of biological and chemical contaminants.
System Configuration:
- Negative-pressure grossing stations fully integrated with exhaust systems
- Dedicated exhaust ducts and independent exhaust fan units
- Room-level negative pressure control (−10 Pa to −15 Pa)
- High air change rate (≥12 ACH)
- HEPA filtration and controlled high-level discharge
Solution Value:
- Minimizes personnel exposure
- Meets infection control and biosafety requirements
- Provides a safe environment for intensive grossing operations
2. Technical Rooms (Processing, Embedding, and Staining)
(Chemical Contamination Zones)
Engineering Objective:
Comprehensive control of VOC emissions from formaldehyde, xylene, alcohols, and other reagents.
System Configuration:
- Integrated design of chemical fume hoods and local equipment capture hoods
- Continuous room negative pressure operation
- Activated carbon or composite chemical filtration systems
- Organized make-up air supply to maintain exhaust efficiency
Solution Value:
- Prevents chemical vapor accumulation and migration
- Ensures environmentally compliant exhaust discharge
- Improves operational comfort and safety
3. Pathologist Offices and Slide Review Rooms
(Clean, High-Value Diagnostic Areas)
Engineering Objective:
Provide a stable, quiet, and clean diagnostic environment.
System Configuration:
- Slight positive pressure control (+5 Pa to +10 Pa)
- 100% fresh air or HEPA-filtered return air systems
- Moderate air change rate (6–8 ACH)
- Precise temperature and humidity control (22–26 °C, RH < 60%)
Solution Value:
- Prevents infiltration of contaminated air
- Protects glass slides and optical instruments
- Enhances diagnostic focus and efficiency
4. Molecular Pathology and Immunohistochemistry Laboratories
(Controlled Clean / Semi-Contaminated Zones)
Engineering Objective:
Prevent nucleic acid amplification contamination and ensure analytical accuracy.
System Configuration:
- Nested pressure gradient zoning (reagent prep → sample handling → amplification)
- Independent exhaust systems for PCR amplification areas
- HEPA-filtered clean air supply
- Cleanliness levels of ISO Class 7–8 (Class 10,000–100,000)
Solution Value:
- Compliance with molecular pathology standards
- Reduced test failure rates
- Support for advanced precision diagnostics
5. Frozen Section Rooms
(High-Risk, Time-Critical Areas)
Engineering Objective:
Ensure biosafety and thermal stability during rapid diagnostic procedures.
System Configuration:
- Dedicated exhaust hoods for cryostats
- Room-level negative pressure control
- Coordinated cooling and exhaust design to manage equipment heat loads
IV. Ventilation System Technologies Supporting the Integrated Solution
- VAV (Variable Air Volume) Control Systems
Real-time airflow adjustment to maintain pressure differentials while reducing energy consumption - System Independence and Redundancy
Separate exhaust systems for contaminated areas, biosafety cabinets, and fume hoods, with standby fan units for critical zones - Exhaust Air Treatment and Compliance
HEPA filtration for biological exhaust and chemical adsorption systems for VOC control - Online Monitoring and Alarm Systems
Continuous pressure differential display with visual and audible alarms - Energy-Efficient and Maintenance-Oriented Design
Optimized for long-term hospital operation and cost control
V. Core Value of the Integrated Engineering Solution
✔ Safety and Compliance – Meets infection control, biosafety, and environmental regulations
✔ System Reliability – Risks eliminated at the design stage
✔ Process Integration – Ventilation systems fully matched with pathology equipment
✔ Sustainable Operation – Energy-efficient, easy to maintain, and expandable
✔ One-Time Construction, Long-Term Confidence
Conclusion
A pathology department is not merely a collection of rooms and equipment—it is a highly integrated life science engineering system.
A truly professional integrated engineering solution ensures that the ventilation system functions as an invisible yet unwavering safety foundation, protecting personnel health and diagnostic integrity at all times.
By establishing a controlled interface between the microscopic cellular world and the broader hospital environment, this solution supports accurate diagnosis, operational efficiency, and long-term clinical excellence.
坤灵最新logo-scaled-1.png)